- Definition: idiopathic proliferation of fibrous & muscular (fibromuscular!) tissue in medium & large arteries. This is not inflammatory and not atherosclerotic.
- FMD is the most common cause of Renal artery stenosis (RAS) in children. If you are looking at a renal arteriogram in a child, think of this first.
- Renal arteries (60%) > ICA / verts (35%) > external iliac (3%) > visceral (2%)
- FMD will involve the cervical Internal carotid artery (ICA), but not the Common carotid artery (CCA) and not the intracranial ICA.
SUBTYPES
(1) medial fibroplasia (85%) = string of beads. MOST COMMON (85%)
(2) intimal (5%) = web-like focal stenosis and post-stenotic aneurysm.
(3) perimedial (10%) = tubular narrowing, irregular stenosis.
(4) mixed
- Digital subtraction angiography findings (DSA): “string of beads”(85%), 50% bilateral
- Treatment: balloon/angioplasty if pressure gradient > 15 mmHg across the stenosis
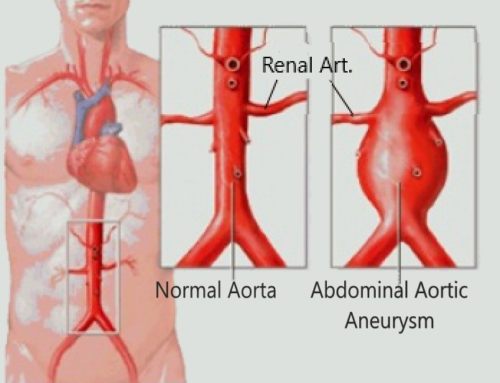
- COMPLICATIONS: aneurysms & spontaneous renal artery dissection (this is almost pathognomonic)
Fibromuscular dysplasia (FMD) is a pathology of the arteries of non-atherosclerotic and non-inflammatory origin, which results in the degeneration of the elastic tissue of the vessels with the simultaneous growth of fibrous and smooth muscle tissues. This leads to narrowing of the lumen of blood vessels and bleeding of organs. Most often, the renal arteries are involved in the process, less often – the aorta, branches of the aortic arch, arteries of the extremities, including the feet and hands. This disease is considered systemic, despite the fact that in each case, the lesion of a certain localization is most commonly seen.
Table of Contents
- Epidemiology
- Pathology
- Diagnostics
- Definition, main stages of study
- Histological changes
- Epidemiological data
- Etiology and hereditary predisposition
- Risk factors
- Localization of FMD in the arteries of the carotid and vertebrobasilar systems
- Clinical manifestations
- Tinnitus and other clinical manifestations
- Cerebral hemorrhages and subarachnoid hemorrhages
- Diaphragm of the bulb of the carotid artery as a form of FMD
- FMD in childhood
- Diagnosing fibromuscular dysplasia
- Treatment and management of patients with FMD
- Outcomes
Epidemiology
Fibromuscular dysplasia is more common in young people and women. FMD is described as the cause of renal artery stenosis in 30% to 60% of children with renovascular hypertention.
Pathology
5 histological types of fibromuscular (fibromuscular) dysplasia have been described (sometimes they are described down to 3):
- Fibroplasia of the media
- makes up 75-80% of all FMD. With it, the tunica media is affected, the areas of its fibrous changes are interspersed with areas of luminal ectasia. This gives a classic angiographic picture of a “string of beads”, in which the alternation of areas of narrowing and swelling is determined, which (the main symptom!) Is larger than the diameter of the artery (i.e. between stenoses – small aneurysms).
- perimedial dysplasia
- less common Pronounced collagen deposits are determined in the outer leaflet of the tunica media. An uneven thickening of the artery wall is noted, and at the same time, there are no areas of aneurysmal expansion in the affected area behind the areas of stenosis (i.e., the diameter of the “bead” is not greater than the diameter of the unchanged artery). In this type, in addition to the development of vasorenal hypertension, there is an increased risk of developing dissections, embolisms, occlusions, and other vascular catastrophes, which is why its identification is so important. A narrow stenosing septum, the membrane may not be visualized even with selective angiography, and in this case (in the absence of an angiographic picture of stenosis in the presence of its reliable DG signs), intravascular ultrasound becomes the “gold standard” as a method for diagnosis.
- Intimal hyperplasia
- less than 10% of all types of FMD. Collagen deposits are found around the inner layer (tunica intima) of the artery wall. It appears concentrically thickened with local rather uniform narrowing (or narrowing) of the lumen on the angiogram (without the formation of a pattern of beads or rosaries).
- media hyperplasia
- Rare variant 1-2%. True hyperplasia of smooth muscle fibers, without local fibrosis of the walls. On angiograms, it also looks like intimal hyperplasia.
- Periarterial hyperplasia
- A rare variant, 1% FMD. Changes in the adventitia, and collagen deposits (fibrosis) are also observed in the perivascular adipose tissue, signs of inflammatory changes in the wall and in the adjacent perivascular tissue.
Diagnostics
Since FMD leads to characteristic changes in the vascular wall. On angiography, it is usually described as a “thread with beads”. The middle or distal segment of the artery usually has a thickened wall. Color Doppler ultrasound reveals high turbulent flow velocity. Color flow mapping helps to identify jagged edges of the affected segment of the renal arteries. The flow rate remains normal in the proximal segment, which remains intact.
Definition, main stages of study
Fibromuscular dysplasia (FMD) is an idiopathic segmental non-atherosclerotic and non-inflammatory disease of medium and small arteries, leading to their stenosis. Although the modern definition of FMD indicates the average and small caliber of the affected arteries, in reality, in our opinion, they should be designated as large and medium, since the carotid, vertebral (VA) and renal arteries, which are most often affected by FMD, are large arteries, and their branches to the middle ones. Although this agreement on nomenclature was reached to unify the terminology in vasculitis, the principle of assessing the caliber of vessels should be general, regardless of the etiology of the disease. Moreover, small (small) arteries, intensively studied by neurologists in recent years, are intraparenchymal,
FMD was first described in 1938 by W. Leadbetter and C. Burkland in a patient with secondary arterial hypertension caused by renal artery stenosis. The term FMD was proposed in 1958 by L. McCormac et al. , who described pathological changes in the arterial wall. In the 1960s and 1970s of the last century, classifications of FMD based on angiographic and histological data were proposed. The greatest advances in the study of the pathophysiology and nature of FMD have been achieved in the last decade. In 2019, the first international agreement on the diagnosis and treatment of FMD was published. It was based on documents on a standardized multidisciplinary approach to FMD developed independently in Europe and the USA (2014), as well as materials from international symposia in 2017–2018..
Histological changes
Pathological changes can be detected in all 3 layers of the arterial wall, according to which there are intimal, medial and adventitial types of FMD. The intimal type occurs in 1–2% of FMD and is characterized by the accumulation of fibrous tissue in the intima with a moderate number of cells. The internal elastic membrane is preserved, but often split. Medial type FMDis the most common (60-90%) and is represented by areas of degeneration of elastic fibers in the media with their replacement by loose collagen, which alternates with areas of loss of muscle fibers and expansion of the lumen of the artery, in the area of which damage to the internal elastic membrane is noted. With predominant damage to the outer layers of the media, damage to the outer elastic membrane is also noted. In rare cases, there is hyperplasia of the myocytes of the media without pronounced deposition of collagen. The adventitial type of FMD is rare and is characterized by collagen deposition around the adventitia with extension into the periarterial tissue and often with concomitant lymphocyte infiltration.
Histopathological classification, distinguishing 3 types of histological changes in the arterial wall, is currently not used in clinical practice according to the international agreement of 2019. This is due to the fact that arterial wall samples are very rarely available for histological examination, since due to the success of endovascular surgery, open vascular surgery is very rarely performed. In addition, the main intravital method for verifying FMD in the clinic is angiography, while correlations between its data and the results of histological examination are not always noted. Finally, different types of FMD can occur in the same segment of an artery, just as they can be found in different arteries in the same patient.
Angiographic classification
There are 2 types of angiographic changes characteristic of FMD: 1) focal FMD, which can be localized in any part of the artery and is represented by concentric (<1 cm in length) or tubular (≥1 cm in length) smooth narrowing of the arterial lumen; 2) multifocal FMD, which is characterized by alternating areas of narrowing and widening of the arteries (bead string syndrome), which are usually observed in the middle and distal parts of the arteries, most often in the renal and carotid. Prior to the adoption of a single international agreement, the European classification used the term “unifocal FMD”, in the American – “focal FMD” . An international agreement has adopted the terms “focal FMD” and “multifocal FMD” .
Angiographic changes characteristic of FMD are often associated with arterial tortuosity, aneurysms, and dissection. These changes are nonspecific and may occur in other vascular diseases, and therefore their isolated detection is not a basis for diagnosing FMD. At the same time, the presence of focal or multifocal changes characteristic of FMD in at least one artery, when combined with an aneurysm, dissection, or tortuosity in other arteries, is considered as a multiple lesion caused by FMD .
Epidemiological data
The prevalence of FMD in the population is unknown. According to pathological studies conducted at the Mayo Clinic, FMD of the internal carotid artery (ICA) occurs in 0.02% of cases. Most often (62-75%) the renal arteries are affected , in 1/3cases involving carotid or PA. Some researchers note that the frequency of lesions of the main arteries of the head (MAH) is currently the same as that of the renal arteries . In 95% of cases of the cerebral form of FMD, the ICA is affected (more often on both sides), in 60–85% of cases it is VA, usually with joint involvement of the carotid arteries . According to the American FMD registry, 64% of patients with cerebral FMD have renal artery involvement, and 65% of patients with renal artery FMD have MAH involvement. According to the European registry ARCADIA (Assessment of Renal and Cervical Artery Dysplasia), 54% of patients with cerebrovascular manifestations of FMD have multiple lesions. Among patients with FMD and cerebrovascular disorders, renal artery damage is 3 times more common in patients with hypertension. Data on the prevalence of damage to other arteries (celiac, brachial, iliac, and coronary arteries) in patients with cerebral FMD are scarce. Spontaneous dissection of the coronary arteries in patients with cerebral manifestations of FMD is rare, while in patients with spontaneous dissection of the coronary arteries, FMD MAH was found in 52% of cases, and intracranial aneurysms in 14%.
FMD is more common (80–90%) in women, but in men the disease may be more severe with the development of aneurysms and dissections. The disease usually manifests itself in the 5th decade of life.
Family history of FMD is noted in 1.9 to 7.3% of patients . These data appear to be inaccurate due to the relative rarity of pedigree studies, incomplete penetrance (~0.5), and the difficulty of diagnosing FMD, especially its subclinical variants .
Etiology and hereditary predisposition
It is assumed that a combination of genetic, exogenous, and endogenous factors is important in the development of FMD. The assessment of the hereditary burden of FMD, as already noted, is difficult due to the frequency of asymptomatic phenotypes and the lack of an accurate characterization of family cases. Studies of FMD candidate genes are ambiguous: in some studies, negative results were obtained, in others, positive results in some cases . Although FMD shares some phenotypic features with monogenic connective tissue diseases such as Marfan, Ehlers–Danlos, and Lois–Dietz syndromes, systematic screening of candidate genes for these syndromes has not revealed an association with FMD. An exome sequencing analysis of 16 FMD patients from 7 families did not reveal gene variants common to all affected sibling pairs .PHACTR1 , which increases the risk of developing the disease by about 40% among carriers of the allele . The locus is located in the intron of the PHACTR1 gene , is associated with the levels of its expression in skin fibroblasts, and can, as shown in the experiment, directly affect the development of blood vessels [24]. Moreover, the same polymorphism regulates the expression of endothelin-1, which affects vascular tone and arterial remodeling . It is important to note that the rs9349379-A allele in the PHACTR1 genes also associated with dissection of the arteries of the neck (ICA/CA), arterial hypertension, and migraine headache, clinical manifestations characteristic of FMD . It also provides protection against atherosclerotic lesions of the coronary arteries . Further genetic studies are needed, since markers specific only to FMD have not been found so far.
Recent studies have found increased secretion of transforming growth factor (TGF)-β1 and TGF-β2 by skin fibroblast cell lines in patients with FMD compared with controls . The levels of TGF-β1 and TGF-β2 were also elevated in the plasma of patients with FMD. The potential involvement of TGF-β pathways in the pathogenesis of FMD is an area for future research. It has recently been suggested that the accumulation of lysophosphatidylcholine (lysoPC), a pro-inflammatory and pro-apoptotic lipid mediator in visceral arteries, may reflect a predisposition to aneurysm development in FMD patients. At the same time, the distribution pattern of lipid molecules, including cholesterol esters and lysophosphatidylcholine, in aneurysms of visceral arteries in patients with FMD and atherosclerosis is different.
Since FMD is considered within the framework of connective tissue dysplasia of a predominantly vascular nature, S. O’Connor et al. conducted a systematic search for signs of connective tissue dysplasia in women with FMD, which could be its potential markers. The authors showed that in patients with FMD, moderately severe myopia, arched palate, crowding of teeth, and early articular syndrome (osteoarthritis) were statistically significantly more common. However, the phenotype characteristic of differentiated syndromes of connective tissue dysplasia was not found. Some features of connective tissue dysplasia, such as arched palate, wide atrophic scars, and pneumothorax, were more common in patients with FMD who had a high vascular risk profile: history of aneurysms or MAH dissections. Formerly L.A. Kalashnikova et al. in sectional cases of dissection of the arteries supplying the brain, dysplastic changes in the arterial wall were found, similar to those in FMD. The study of skin and muscle biopsies in patients with ICA/VA dissection revealed a systemic nature of dysplastic changes in the connective tissue. Assessment of clinical signs of connective tissue dysplasia in patients with dissection of the ICA and VA, manifested by ischemic stroke (IS) or isolated cervicocephalic syndrome, revealed them in 54% of patients .
Risk factors
Smoking is a potential pathogen associated with FMD. A case–control study showed an association between current smoking (OR, 2.5–4.05) or previous smoking (OR, 1.8–4.1) and renal FMD. According to the American FMD Registry, the incidence of aneurysms is significantly higher in former smokers than in never-smokers, in addition, they tend to have a higher frequency of vascular episodes. Despite these data, smoking cannot be considered as a necessary condition for the development of FMD.
Exposure to endogenous or exogenous female sex hormones is also associated with FMD. Although the disease is more common in women, a clear causal relationship has not been established for the development of FMD or its complications in patients taking contraceptives or other female sex hormones. It has recently been shown that patients with FMD have an imbalance between estrogen and progesterone receptors, with the latter being intensely expressed in the nuclei of smooth muscle cells of renal artery samples obtained from patients operated on for the renal form of FMD. In the control group, this imbalance was absent . These results suggest that progesterone may also play a role in the pathogenesis of FMD, which needs further research.
Localization of FMD in the arteries of the carotid and vertebrobasilar systems
FMD most often affects the cervical part of the ICA and VA, involving the middle and distal parts of the ICA and VIII-VIV segments of the VA at the level of C I – IIvertebrae. The intracranial regions are rarely affected in adults, while their involvement is common in children. In adults, intracranial lesions are usually a consequence of the spread of FMD from extracranial arteries . Isolated lesions of the intracranial arteries (distal ICA, middle cerebral artery, basilar artery) with a typical angiographic sign of “strings of beads” are rare . It is possible that intracranial lesions in FMD are actually more common. This assumption is based on reports of detection of FMD only during histological examination of arteries in cases with a fatal outcome. Histological changes in the arteries, characteristic of FMD, were also observed in our cases of intracranial dissection of the ICA, which ended in death, while macroscopically typical for FMD changes were absent. Histological signs of FMD have also been described in fusiform or dolichoectopic changes in the intracranial arteries, especially the basilar artery, moyamoya syndrome, and carotid-cavernous fistula. These observations raise the question of the obligatory nature of the angiographic sign “thread of beads” in the diagnosis of FMD.
Clinical manifestations
The most common manifestations of FMD are headache and tinnitus. Transient cerebrovascular accidents (TIMC), IS and hemorrhagic strokes, subarachonoid hemorrhages are much less common. FMD is often clinically asymptomatic and is an incidental finding on angiography performed for another reason.
Headache is observed in 70% of patients with cerebral FMD, and in 30% of cases it is represented by migraine. Despite the high prevalence of headache, its pathophysiological mechanisms remain unclear. The role of turbulent changes in blood flow in the arteries supplying the brain, increased pain sensitivity of the dura mater, and increased blood pressure are suggested . Almost 1/2 of patients with spontaneous dissection of the ICA and VA, which, according to our data, is based on arterial wall dysplasia, have a history of headache. At 2 / 3cases, it does not meet the criteria for migraine, and according to a neurophysiological study, it is not associated with hypersensitivity of neurons in the cerebral cortex, which is characteristic of migraine as an independent disease . It is assumed that dysplastic changes in the craniocerebral arteries play a role in its genesis .
Tinnitus and other clinical manifestations
Pulsatile tinnitus , often described by patients as “whistling” in the ears, occurs in 40% of patients with FMD MAH. Its origin, apparently, is associated with a violation of the laminar blood flow in conditions of an altered arterial lumen, represented by areas of stenosis and expansion due to a change in the wall of the arteries. Noise can be heard during auscultation in the projection of the ICA on the neck. Less common symptoms include neck pain or carotidynia, blurred vision, dizziness, and pre-syncope. They may be based on cerebral hypoperfusion due to morphological changes in the arterial wall and impaired response of autoregulation of cerebral blood flow.
IS and MIH are one of the complications of FMD of the arteries of the neck. The frequency of IS is 10%, and MI is 20%.
Dissection of the ICA or VA is the main cause of cerebrovascular accidents, and it is more common in patients with FMD than in the general population. According to the US FMD registry, the frequency of ICA dissection among patients with FMD is 16%, and VA dissection is 5%. %), the frequency of multiple dissections reaches 37%.
According to the European registry ARCADIA (Assessment of Renal and Cervical Artery Dysplasia), the frequency of MA dissection in patients with FMD and neurological manifestations is 27%. The frequency of FMD among patients with MAH dissection can reach 15–20%, increasing in the presence of multiple dissections of the arteries of the neck.
Among our almost 300 patients with ICA/VA dissection, verified by MR and/or CT angiography, less often by subtraction angiography, clear-cut changes in their walls, characteristic of FMD, were absent in all patients, and clear-cut changes in the renal artery were found in 1 patient with repeated dissections, who underwent angiography of the renal arteries due to renal infarction. Despite the absence of changes in the ICA/VA characteristic of FMD during angiography, we believe that FMD is the main cause of spontaneous dissection of the ICA/VA, since morphological studies in cases with a fatal outcome from severe ischemic stroke revealed dysplastic changes in the arterial wall, similar to those with FMD.
Clinical manifestations of IS in FMD do not differ from those in spontaneous dissection of the ICA/VA, which are described in detail in Russian literature.
In addition to dissection, other causes of MI or IS in patients with FMD may be stenosis or occlusion of the MA, which leads to cerebral ischemia by a hemodynamic mechanism; arterio-arterial embolism with thrombi formed in the area of stenosis or dilatation of the arteries; occlusion of perforating arteries secondary to arterial hypertension, which many patients with FMD suffer from due to damage to the renal arteries (lacunar infarcts).
Cerebral hemorrhages and subarachnoid hemorrhages
Cerebral hemorrhages rarely develop in patients with FMD. They are caused by microangiopathy due to arterial hypertension, rupture of intracranial aneurysms, or dissecting intracranial aneurysms. Subarachnoid hemorrhages are also caused by rupture of aneurysms, less often by dissection of the intracranial segment of the vertebral artery. The frequency of subarachnoid hemorrhages among patients with FMD is 3%, among patients with acute neurological disorders it is 20%. Some authors recommend intracranial CT or MR angiography to exclude aneurysms in patients with confirmed FMD in at least one arterial system, since their frequency in this category of patients is higher than in the general population . In contrast, a meta-analysis published in 1998 and included 498 patients with cerebral FMD, found unruptured intracranial aneurysms in 7% of cases, which is slightly higher than in the general population (<5%) and significantly less than reported in the 70-80s of the last century ( 22–51%) . According to the American FMD registry , 13% of women with this disease had at least 1 intracranial aneurysm, and 4% had more than 1. Almost inIn 1/2 cases , the size of aneurysms exceeded 5 mm, which is associated with a high risk of their rupture.
Diaphragm of the bulb of the carotid artery as a form of FMD
The disease, described under the name “carotid web”, or “diaphragm of the bulb of the carotid artery”, is classified by some authors as an atypical form of FMD. The disease is rare, predominantly in representatives of the Negroid race and residents of the Caribbean . Most often, middle-aged women get sick. Diaphragms are thin translucent intravascular membranes located in the lumen of the ICA (mainly along the posterolateral wall of the bulb of the carotid artery), less often in the VA (orifice or segment VIII), which are visualized during MR/CT angiography as a linear defect that does not change and does not disappear when the position of the patient’s head changes . Descriptions of morphological changes in diaphragms are few. Histological examination reveals edematous tissue with a loose matrix and individual spindle-shaped cells, especially in the area of outgrowth, which leads to intimal hyperplasia. The relationship of this formation with the classical form of FMD is not clear, especially since similar defects in other arteries have not been described. In addition, histological examination of the diaphragms in some cases reveals signs of atheroma .
The main clinical manifestations are MIH or IS. It is assumed that they develop according to the mechanism of arterio-arterial embolism. Emboli can form above the diaphragm due to decreased blood flow or prediaphragmally, in an aneurysmally dilated bulb . Another mechanism may be dissection of the arterial wall. In the presence of PNMK or IS, the frequency of their recurrence, even in patients receiving antiplatelet therapy, reaches 30% per year , which justifies endarterectomy or stenting .
FMD in childhood
The cerebrovascular form of FMD in children (<18 years) differs from that in adults in both clinical manifestations and histological characteristics. With the same frequency, the disease occurs in boys and girls, and in most cases is diagnosed in early childhood (average age 7 years), and in 1/3cases – in the 1st year of life. The predominant form is focal FMD, which histologically corresponds to intimal fibroplasia, while typical adult multifocal FMD, which is histologically usually characterized by medial fibroplasia, is rare . A combination of FMD with moyamoya syndrome and intracranial aneurysm has been described. The cerebral form of FMD in 63% of children is combined with damage to the renal arteries, and in 72% – with the involvement of additional arterial systems. The main clinical manifestation is IS (63%), which is most often associated with intracranial artery stenosis and can occur in several territories (40%). The prognosis is poor with a high annual rate of recurrent stroke (10%) and mortality (13%) .
Diagnosing fibromuscular dysplasia
Digital subtraction angiography remains the “gold standard” for diagnosing FMD, as it has a higher sensitivity than CT or MR angiography in detecting changes characteristic of FMD — the “string of beads” symptom . Subtraction angiography is commonly used in cases of FMD requiring endovascular treatment, as this study in FMD is associated with an increased risk of iatrogenic dissection compared with the general population. That is why the main method of imaging in most diagnostic centers is CT or MR angiography with the introduction of contrast.
Duplex scanning is less informative than CT or MR angiography . It can be used to diagnose and monitor patients with FMD in specialized centers with experience in ultrasound in this pathology. In addition, ultrasound does not allow assessing the state of intracranial arteries .
Treatment and management of patients with FMD
General recommendations include quitting smoking, as it is associated with an increased incidence of intracranial aneurysms; limitation of physical activity and sharp head turns, refusal of manual therapy, which increases the risk of developing dissection of the ICA and VA . The issue of women taking hormonal drugs remains open, since there are no data in favor of both their safety and the development of complications.
Drug treatment has not been evaluated in randomized placebo-controlled trials in FMD. Treatment approaches are based on the description of individual clinical cases, expert opinion (empirical recommendations), taking into account known morphological changes in the arteries and pathophysiological mechanisms. The combination of arterial narrowing and dilatation, which is characteristic of FMD, creates favorable conditions for thrombosis, justifies the use of antiplatelet agents, the indications for which increase in old age and in the presence of concomitant risk factors . With an increased risk of bleeding, antiplatelet agents are not prescribed.
In uncomplicated FMD (without CIMC/IS), as well as in asymptomatic MAH dissection, daily low (75–100 mg/day) doses of aspirin are recommended. The advisability of additional prescription of alternative antiplatelet drugs or their combination for the purpose of primary prevention of stroke has not been proven . The presence of small clinically asymptomatic intracranial aneurysms characteristic of FMD is not a contraindication to aspirin. In general, there are insufficient data on the relationship between the use of antiplatelet agents and the risk of rupture of intracranial aneurysms .
It is necessary to control arterial hypertension, which is often observed in patients with FMD due to concomitant damage to the renal arteries .
Statins are not indicated in patients with FMD, since, according to a study of patients with MAH dissection, one of the causes of which is FMD, there is an inverse relationship between dyslipidemia and the risk of dissection . The appointment of statins should be considered only in the presence of concomitant atherosclerotic lesions of the arteries.
Surgical or endovascular treatment is not recommended for patients with asymptomatic cerebral FMD, regardless of the severity of stenosis.
Specific studies on the treatment of headache, one of the main neurological manifestations of FMD, which is often interpreted as migraine, have not been conducted. For the interruption of a headache attack and its prevention, the same approaches are recommended as for migraine in general. Triptans, ergotamine, and other sympathomimetic agents should be used with caution due to the potential for vasoconstriction . In addition, the elimination of factors provoking a headache attack and lifestyle modification are recommended .
Experience with the treatment of pulsatile noise in FMD is limited. Consultation with an otorhinolaryngologist and an audiologist is recommended. Psychotherapy, cognitive-behavioral therapy, sound therapy are carried out .
Treatment approaches for unruptured intracranial aneurysms in FMD are the same as in the general population . Smoking and alcohol cessation is recommended . Predictors of growth and rupture of aneurysms are unknown, and therefore their monitoring is necessary using neuroimaging, the frequency of which has not been determined.
The treatment of MIH and/or IS in patients with FMD is similar to that in patients without FMD. In the acute period of a stroke, according to general indications, intravenous systemic thrombolysis and / or mechanical thrombolysis can be performed. Patients who “go beyond” the therapeutic window are prescribed antithrombotic drugs . Secondary prevention of MICC and/or IS includes long-term antithrombotic therapy.
Endovascular treatment is indicated for patients with repeated episodes of cerebral ischemia despite optimal drug therapy . Dissecting MA aneurysms as a complication of FMD have a low risk of cerebral ischemia and rarely require endovascular treatment . Information on endovascular or surgical treatment for intracranial dissection or moyamoy syndrome in patients with cerebral FMD is limited. When performing endovascular intervention in patients with FMD, care should be taken due to the risk of dissection in case of damage to the vascular wall.
Outcomes
According to long-term follow-up of patients with cerebral FMD, most of whom had CIMC and/or IS, the risk of their recurrence is 0.6–3.7% per year, and intracerebral hemorrhage is <1% per year. Recent studies of prognosis in FMD with MA involvement have shown that the annual recurrence rate of stroke or MIMC is less than 0.5% . According to the American Registry, FMD, MIH and IS mainly occur in patients with concomitant MAH dissection . The risk of progression of FMD at the extra- or intracranial level is <1% per year, however, systematic neuroimaging screening was not performed in the studies that presented these data


Leave a Reply