-
Table of Contents
Quick Take:
- Symptoms: facial/neck edema, Jugular vein distention (JVD), headache. Potentially life threatening if progression to laryngeal edema.
- Etiology/Causes: lung cancer (80%), catheter-related stenosis/thrombosis, fibrosing mediastinitis (histoplasmosis, Tuberculosis (TB), etc.)
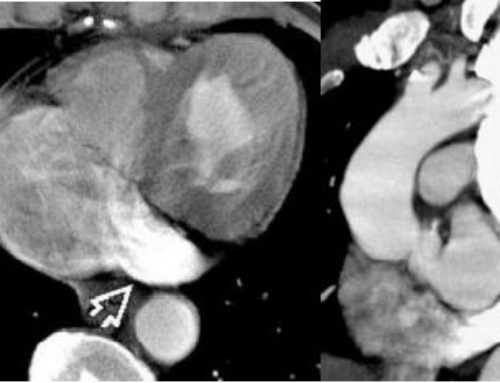
- Contrast Enhanced CT imaging findings: occluded veins most commonly the superior vena cava (SVC), you may also se a hot quadrate lobe = enhancement of Segment IVb, collateral veins
- Treatment: catheter-directed thrombolysis, followed by balloon angioplasty and stenting. After stenting, recommend Plavix
Introduction
SVC syndrome results from the obstruction of blood flowing from the head, neck, arms and face to the heart. This blood from the upper extremity converges and follows the path through the Superior Vena Cava (SVC) before returning to the heart. The obstruction of the superior vena cava can occur as a result of various pathologies but regardless of the underlying cause SVC syndrome has a typical clinical presentations with the most common cause of obstruction today being lung cancer invading into the superior vena cava or mediastinum. Historically in the 18th century, syphilis and tuberculosis were responsible for about 40% of the known cases of SVC syndrome. The clarification of the hemodynamic changes in this syndrome as well as the availability of diagnostic imaging and cytopathology techniques today allow doctors to treat this syndrome more safely and effectively than was possible just 10 years ago. Contrast CT and MRI assist in diagnosing the location of the obstruction and more invasive techniques such as cavography/venography can delineate the level of obstruction and vessels involved. The diagnosis of SVC obstruction and the various imaging techniques greatly enhance the ability of physicians to diagnosis and identify treatment options such as percutaneous transluminal angioplasty. In some patients radiation oncology can allow for non invasive treatment obstruction due to a mass with radiation fractionation technique that today allows high doses of radiation to be administered to patients with high levels of accuracy directing therapy at the malignant neoplasms, with approximately 70% of patients seeing significant benefits in tumor burden reduction.
History of SVC Syndrome
Superior Vena Cava Syndrome (SVCS) was first described before the advent of antibiotics. In 1757, the first record of a patient with the syndrome was published by William Hunter. This case report referred to a patient with syphilis who had developed syphilitic aneurysm of the thoracic aorta. Infectious diseases such as syphilis and mediastinal tuberculosis were the causes of 40% of cases in the series of 274 reviewed and published by Schedter in 1954. Currently, lung carcinoma is responsible for 70% of cases, malignant diseases of the mediastinum and fibrosis as well as catheter-related thrombosis are the majority of the remaining causes. Today only 5% of the causes are benign in nature, the incidence appears to have increased, but this percentage has not been well established.
Signs And Symptoms
Superior Vena Cava Syndrome (SVCS), by definition, describes the clinical presentation of obstruction to blood flow in Superior Vena Cava (SVC). The clinical syndrome has a gradation that is correlated with the degree of vein obstruction in the mediastinum. Detecting the initial signs of the syndrome, such as morning facial edema, is commonly done by pulmonologist or thoracic surgeons. Facial edema is an important symptom in helping identify the final diagnosis. Additionally other methods of diagnosing cause of an obstructive mass include needle aspiration, lymph node and mediastinal biopsies.
Dyspnea(Shortness of breath), facial swelling and cervicofacial edema are the most frequent symptoms and up to 60% of patients arrive at the doctor’s office with these complaints. Cough, swelling of the upper limbs, chest pain and dysphagia (Difficulty swallowing) may appear.
Cough-syncope is a symptom commonly attributed to patients with COPD but can also manifest in SVC syndrome patients where fits of coughing can result in fainting. This can sometimes even be the first manifestation of the syndrome. Cough-syncope etiology and pathogenesis is debated and can be explained by decreased venous return to the heart during coughing fits or by the equalization of pressures in the arterial and venous capillaries within the brain during coughing resulting in decreased blood flow to the brain as venous drainage is compromised resulting in loss of consciousness. Transient ischemia and syncope can occur. Some patients, may experience episodes until collateral circulation relieves venous hypertension.
The appearance of collateral circulation is dependent on the degree of obstruction of the SVC, it is usually most prominent in complete occlusion of the vessel/SVC. Collateral venous flow includes venous drainage through the azygos veins, internal or mammary, lateral thoracic veins, as well as the paraspinal veins and the periesophageal venous plexus. These vessels also become engorged in an attempt to find a way for blood to return from the upper extremities to the heart when the superior vena cava is obstructed. In these patients when attempting to find venous access sites for infusions, the veins chosen should be those of the lower limbs. Infusions in the veins of the upper or cervical limbs may worsen the clinical picture. Peribronchial edema and cerebral edema can worsen with increased pressure caused by infusions administered to the upper extremities.
Neurological symptoms can also be present and are related to the degree of existing cerebral edema. Syncopes may appear after coughing, dizziness, headache and the sensation of facial enlargement. The elevation of the head of the bed, nasal oxygen as well as diuretics and corticosteroids can help.
Diagnosing SVC syndrome
The diagnosis of superior vena cava syndrome must be established through history and physical examination. Symptoms and patient history are likely the most important ways to diagnose the disease. For example symptoms such as “getting up in the morning with a swollen face”, and neck edema/swelling without other explanations, are important clues. Engorgement of neck veins or formation of visible collateral veins on the chest are also important clues. Additionally loss of consciousness with coughing fits is another clue .
Once the clinical diagnosis is suspected or established, complementary exams should be performed including chest X-rays in frontal and lateral views, computed tomography or magnetic resonance imaging. Imaging exams such as xrays of the chest may initially point to mediastinal enlargement due to the presence of a mass or lymph node enlargement. Venography is generally reserved for patients contemplating endovascular treatment and to show the level of the obstruction. The location of obstruction may be at the junction of the brachiocephalic trunks, in the subclavian veins, in the jugular veins or in the vena cava itself.
The presence of calcified lymph nodes in the mediastinum points to the diagnosis of histoplasmosis or other granulomatous infections which can cause these lymph nodes. It should be noted that normal appearance of the mediastinum on the initial chest X-ray does not exclude mediastinal fibrosis or several other causes of superior vena cava syndrome .
Imaging such as CT or MRI can help identify relationship of a mediastinal mass to the SVC. Tumors and mediastinal masses should be studied for their relationship with SVC, including extrinsic compression, minimal invasion, partial or total invasion of the vessel. This information is also essential for thoracic surgeons to be able to identify patients who can undergo primary surgical resection. Simple vessel compression does not remove the possibility of surgical resection in cases resulting from lung cancer. However most surgeons will consider mass involvement of greater than 180 o circumference of the nearby vessels as an exclusion criterion for radical resection.
In most situations biopsies are required once the mass has been identified on imaging to help confirm the etiology. This can generally be done by percutaneous CT guided biopsy of the mediastinal mass but on occasion masses causing superior vena cava syndrome cannot be sampled by a percutaneous route and will require mediastinoscopy for biopsy.
Causes of SVC syndrome
Lung cancer is responsible for 70% of the cases of SVCS. Of a series of 4,100 lung cancer cases reported in the literature, 2.4% of patients had SVCS. Undifferentiated small cell carcinoma is the most common histological type, appearing in up to 38% of cases. Lymphomas account for 8% of cases of SVCS, with the sclerotic type being the most frequent. Hodgkin’s lymphoma frequently involves the mediastinum, but rarely causes the syndrome. Thymomas and germ cell tumors also account for a small percentage of cases. Leukemia is a rare cause of this syndrome. Metastatic breast carcinoma is the most frequently seen cause among secondary neoplastic causes of superior vena cava syndrome. Among the non-malignant causes, mediastinal fibrosis secondary to histoplasmosis seems to be the most frequent. Large substernal thyroid goiters have been reported to cause superior vena cava syndrome; compression of the vein in the mediastinal space between the trachea and the sternum can lead to compression of the superior vena cava. Catheters often used for dialysis including long term tunneled hemodialysis catheter which enter through the jugular vein but traverse and sometimes terminate in the superior vena cava, as well as the Swan-Ganz catheters and also pacemaker leads, can lead to thrombosis and resultant superior vena cava syndrome. Other rare lesions such as hemangioendothelioma and Behçet’s disease can cause SVC occlusion.
Granulomatous diseases of the mediastinum can cause SVCS by increasing the size of the mediastinal lymph nodes resulting in compression of the vein.
Treatment Options
The classic treatment of SVCS includes the use of clinical measures such as elevation of the head of the bed, oxygen, diuretics and corticosteroids, aiming to decrease the edema drained by vena cava and thus reduce the neurological and respiratory symptoms present in this syndrome. The use of venous access only in the lower limbs serves to prevent not only the worsening of the symptoms of venous hypertension, but also allows injected medicine a longer time to distribute in the systemic circulation.
The treatment of the syndrome requires establishing the etiology of the diagnosis; patients’ clinical conditions may not always sich the diagnosis. Different etiologies such as lymphoma have significantly different treatment options some of which include medication and others of which may require radiation.
Some have advocated for grafting the superior vena cava to treat superior vena cava syndrome but this has not been able to demonstrably extend survival. Some specific situations, such as in cases of invasive thymomas initially treated by chemotherapy and / or radiotherapy, may have in superior vena cava grafts placed as an adjunct to the surgical resection of the mass.
Resection of mediastinal tumors and replacement of SVC with a graft is only indicated when there is curative intent. Palliative surgery is generally not supported because of the significant morbidity. In the presence of extensive and developed collateral circulation, graft surgery is generally contraindicated due to the risk of thromboembolism.
When indicated the most commonly performed graft/replacement of the superior vena cava is performed between the innominate or jugular vein on the left side and the right atrium, using a prosthesis in an end-to-end anastomosis. The most commonly used prosthesis is PTFE (polytetrafluoroethylene). The prosthesis is sutured initially next to the atrium and then to the chosen upper vein. PTFE commonly used is number 18 or 20, and its essential that patients be properly heparinized, anticoagulation must be maintained. monitoring and maintenance of central venous pressure prevents thrombosis .
Other techniques include autograft which is when tissue is transplanted from another portion of the body to create a graft often done with pericardium or a spiral graft using the autologous saphenous vein. These methods are less often used than PTFE because they increase operative time and procedural complications.
Percutaneous transluminal angioplasty performed by a trained interventional radiologist or an endovascular trained vascular surgeon is used to treat catheter-induced thrombotic obstructions. This procedure is performed by inserting a expandable metallic stent or balloon mounted stent capable of opening the SVC, thus maintaining blood return back to the heart from the head/neck and upper extremity. The Wallstent and Gianturco Z stent are used and proper deployment can result in 93% of symptom relief .
In catheter induced thrombosis the removal of the catheter combined with anticoagulation is another viable alternative or first line treatment option.
In patients with an established diagnosis of non-small cell lung cancer, irradiation treatment should be sought in cases of SVC syndrome. Symptomatic relief is frequent in this patient group with an approximate two years of survival of 2%; the one-year survival after irradiation can reach 17%. Despite the low survival in these patients the quality of life improvement with this treatment is significant. The fractioned irradiation technique is the most used in this setting. It includes two to four initial fractions of 300 to 400cGy, followed by conventional fractionation up to a total dose of 3,000 to 5,000cGy. There is clinical improvement in 70% of patients with this type of regimen.
Summary
- Superior vena cava (SVC) syndrome results from obstruction of blood flow in the SVC. The majority of cases are due to due to lung cancer or non-Hodgkin lymphoma (NHL). In addition, patients with malignancy have a higher risk of venous thrombosis related to indwelling venous devices (eg, central venous catheter, pacemaker).
- Symptoms and signs of thoracic central venous obstruction can include facial or upper extremity edema, chest pain, respiratory symptoms, or neurologic manifestations. Regardless of etiology, dyspnea is the most common presenting symptom.
- A grading system has been proposed that stratifies symptoms based on severity and also informs the approach to diagnosis and treatment. Although other grading systems are in use, in general, we prefer this grading system as it informs the diagnostic and therapeutic pathway.
- The approach to imaging depends on the time course of the presentation and symptom severity
- For patients with severe or life-threatening symptoms, venography provides the most expedient diagnosis. Following initial stabilization (secure airway, support breathing and circulation), we prefer catheter-based (standard) venography rather than computed tomography (CT) venography because it provides an avenue by which to provide immediate treatment to alleviate SVC obstruction using endovenous techniques (ie, pharmacologic thrombolysis or mechanical thrombolysis) with SVC stenting, as necessary. Further evaluation to determine the cause of the obstruction (if not apparent on venogram) can be obtained once symptoms have improved. CT venography may be an alternative initial imaging modality, depending on the clinical scenario and institutional availability of resources.
- Duplex ultrasound is useful for excluding thrombus in the subclavian, axillary, and brachiocephalic veins and is the initial imaging study for patients with mild symptoms who have an indwelling device or a malignancy at low risk for causing SVC syndrome.
- For patients with clinical features suggestive of mild to moderate SVC syndrome who have a known malignancy that is associated with SVC syndrome, cross-sectional imaging (contrast-enhanced CT, magnetic resonance imaging [MRI]) is a more appropriate initial study.
- Approximately 60 percent of patients present with SVC syndrome without a preexisting diagnosis of cancer. If the imaging studies are consistent with a malignancy, a histologic diagnosis is required prior to initiating specific antitumor therapy.


This is the best information I have found on SVC Syndrome. I have this and not much information the doctor could give me about it. I wanted to understand it and how to go about alleviating some of the symptoms.
I do have a question. After being treated by placing a SVC stent and Removing thrombosis and being put on blood thinners, can the syndrome come back?
Hi Barbara. The answer to that question depends on the cause of SVC syndrome. Having said that SVC syndrome can generally recur especially if the underlying cause is not addressed.